ADVERTISEMENT
Dietary Supplements
The budget authority total, which is significantly higher than the amount floated in an April OMB draft document, suggests there may have been successful lobbying to increase FDA funding.
Some changes the administration has proposed are a “great example of regulating by press release,” says Duffy MacKay, CHPA’s dietary supplements chief. But “after 30 years, the Dietary Supplement Health and Education Act could be modernized to serve the consumer better.”
Result of firms marketing weight loss products to Black and Latino girls and to lower-income households is to “worsen health inequities by gender, race, ethnicity and income,” says Harvard researcher Bryn Austin, director of Strategic Training Initiative for the Prevention of Eating Disorders.
“We've made some progress and really hope to see some movement as we move forward this year,” says FDA Office of Dietary Supplement Programs director Cara Welch.
Trump’s first-term public health appointees “were serious people doing serious work in a bipartisan nature,” but HHS Secretary Robert F. Kennedy and other current presidential advisors “are not serious people,” says Massachusetts’ Jake Auchincloss.
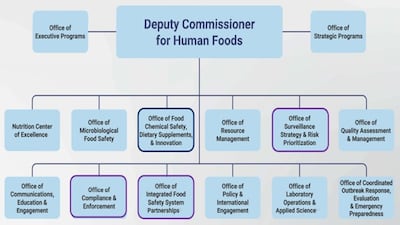
Among FDA's diversions from accepted practices is not going “through the bureaucracy” of advisory committees for experts’ input on potential changes to improve the safety and nutrition profile of food products, says Commissioner Martin Makary.
“It was a little hard to begin after the change or the reduction in force. We're doing everything we can to do an assessment, restore some services of individuals, and also try to rebuild the culture, because it is a great culture,” says FDA Commissioner Martin Makary during FDLI conference.
NAD says Amazentis didn’t substantiate cellular performance and muscle function claims for its supplements containing proprietary ingredient Mitropure but provided “a sufficiently reliable and reasonable basis” for “clinically proven to revitalize mitochondria.”
Same-day announcements of RFIs from HHS secretary cover nutrient review process for infant formula and Kennedy’s “10-to-1 deregulatory policy” as part of president’s “broader federal effort to reduce regulatory burdens and increase transparency.”
Former FDA OTC division director joins CHPA; PLT Health names global sales chief; and Kenvue loses CFO to Mattel, hires replacement from Kellanova.
Potential for supply disruption coupled with manufacturers’ costs for moving supply chains to US emphasized by industry trade groups in comments submitted to Department of Commerce on whether pharmaceutical and dietary ingredients should remain exempted from tariffs President Trump has imposed.
As largest provider of private label/store brand OTCs with “100 plus molecules across 100% price point coverage,” CEO Patrick Lockwood-Taylor says Perrigo’s in position to capitalize as consumers become “more cautious” and look for lower-priced products.